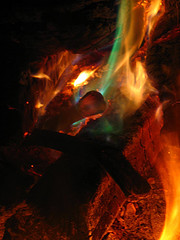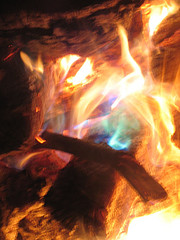11/22/12 Update Note:
This article was thrown up in 2007 after a quick Google research project and it appears that the campfire temperature figure of 2000K was pretty off! The source was a high school teacher’s website as you can see below. Well, that was what was available at the time. There are several other Google listed results now for campfire temperature that seem to put the figure much lower. The campfire clearly didn’t melt the copper and therefore gaseous reactions on the copper side are unlikely, so what explains the color?
It turns out that Chlorine gas (released when burning rubber) reacts with Copper to form Copper Chloride, which is a brownish-yellow solid. Upon reaction with water, Copper Chloride forms, which creates blue and green-colored liquids/crystals.
“Copper(II) chloride is also used in pyrotechnics as a blue/green coloring agent. In a flame test, copper chlorides, like all copper compounds, emit green-blue.” Wikipedia http://en.wikipedia.org/wiki/Copper(II)_chloride
So there you have it – some combination of copper and copper chlorides likely are putting on the show.
______________
So I learned a new campfire trick this weekend that has awakened the dormant chemical engineer in me. I am determined to get to the bottom of this one so my research is below. The trick is that if you buy a tube of copper and insert it into a garden hose (or any other kind of rubber hose, so I was told), and toss it into the campfire, it will create a dazzling light show of greens and blues in the campfire. I believe my hosts inserted a black rubber hose into a copper tube sealed on one side…same effect.
Below is an example of some of the colors.
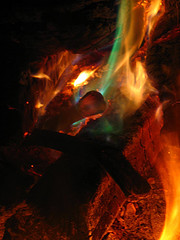
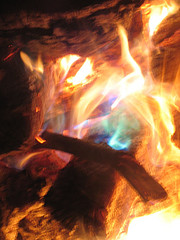
I was immediately curious about which specific chemicals were involved, and naturally, what heath concerns there were to be had.
All I was able to observe was that there was gas produced by a reaction (the colors did not stem right from the copper tube, they were spread over the whole fire), and you don’t get the normal “burning rubber” smell when it burns in this way. Some people will also bore holes into the copper pipe which supposedly helps the process along.
Here’s my research so far:
Colors: Green & Blue Flames
Campfire Temperature: Up to 3140 degrees Farenheit, hot enough to melt copper and cause many gaseous reactions
Main chemicals involved in producing color: Copper with Chlorine produces blue flames, Copper itself will give off green light when very hot
Health Concerns: Burning anything that contains chlorine/chlorinated plastic will create poisonous gases that will lay the foundation for cancer in your body. Burning rubber/plastic = BAD. Don’t do it every weekend.
Rubber Hose Composition:
“The garden hose material that you seek is to make this colored flame work well is poly vinyl chloride (PVC). When combined with the copper it will produce very lovely flames. When you burn this material it releases several gases. One of those gases being phosgene gas. Perhaps you have heard of this gas. It was quite popular during World War I where it was used as a chemical weapon. I will take my campfires without any nerve gas please.”
http://campingearth.com/blog/2007/03/17/turn-a-boring-campfire-into-a-colorful-light-show/
“The majority of garden hoses are made of one of four types of materials: rubber, polyurethane, vinyl, or recycled rubber. Vinyl hoses are probably the least expensive hoses but they have the shortest life span. Rubber and reinforced rubber hoses are slightly more expensive and also more flexible, hence they will most likely outlast a cheap vinyl hose. A hose with an added layer of outer cord reinforcement (made with nylon or rubber) will best resist abrasion and wear. Hardier, reinforced hoses can withstand weather changes and punctures.”
Rubber Research:
“Indeed, burning tires spew deadly chemicals into the air, including hydrocarbons, dioxins, hydrogen chloride, arsenic, nickel, zinc and chromium.”
http://findarticles.com/p/articles/mi_qn4191/is_20000812/ai_n9973511
“It may come as a surprise to some to learn that most garden hoses leach lead and are unsafe for drinking. Warning labels accompany these hoses in many cases, but not all. A lead-free garden hose will clearly be marked as safe for drinking. These hoses are often sold as marine or recreational vehicle (RV) hoses. While there can be minute amounts of lead found even in tap water, hoses made with polyvinyl chloride (PVC) or brass fittings can leach unsafe levels of lead into the water. Lead is used in the manufacturing process of brass fittings to make the brass malleable in order to shape it, and is also used as a stabilizer in PVC. According to a May 2003 article from Consumer Reports, the Environmental Protection Agency (EPA) deemed safe levels of lead to be less than 15 parts per billion. Consumer Reports tested 16 of the most popular hoses sold nationwide, finding that many leached up to 100 times that amount at the initial flush of standing water. http://www.wisegeek.com/what-is-a-lead-free-garden-hose.htm
Campfire Temperature: “A camp fire is ~ 2,000K” (That’s about 3140 degrees Farenheit) http://www.newton.dep.anl.gov/askasci/gen06/gen06272.htm
Plastics Research:
Burning plastics is also very very bad for you. Backyard trash burning is thought to be one of the biggest sources of dioxin poisoning.
“Some types of plastic contain elements besides the standard carbon,hydrogen, and oxygen. Nylons contain nitrogen, and polyvinyl chloridecontains, of course, chlorine.”
http://www.newton.dep.anl.gov/askasci/chem00/chem00031.htm
Copper Research:
From the Cupric Chloride MSDS:
“copper fume…can cause symptoms similar to the common cold, including chills and stuffiness of the head.”
http://www.jtbaker.com/msds/englishhtml/C5863.htm
“Copper (I) chloride salts imparts a blue colour to flames. The picture above shows the colour arising from adding cuprous chloride (CuCl) to a burning mixture of potassium chlorate and sucrose. This flame is relatively cool. Hotter flames burn green bacause of emission from copper atoms (only to be demonstrated by a professionally qualified chemist following a legally satisfactory hazard asessment).”
http://www.webelements.com/copper/
“When you heat copper ions, the energy “excites” electrons and pushes them into higher energy levels. Being in this excited state is not particularly stable, so the electron rapidly returns to the ground state and emits a photon (a particle of light) to release the energy it gained by being excited. These photons are the green light you see from excited copper atoms returning to the ground state.”
http://van.physics.uiuc.edu/qa/listing.php?id=2496